Single Cell RNA-seq Analysis with Bioconductor
Overview
During the lab session we practiced analyzing 10x Genomics data starting with output files from the 10x Genomics Cell Ranger pipeline. While this demonstrates how you might analyze new data that you generate, there are many public datasets that you may wish to explore starting with the analysis already conducted by the original authors. In this assignment, you will obtain data from the Fly Cell Atlas which characterized 15 tissue types alongside whole head and body. Through a coordinated effort among >40 Drosophila labs around the world, more than 250 single-cell clusters were identified and annotated. After loading and exploring the quality of the Gut dataset, you will identify marker genes to gain insights into biological functions.
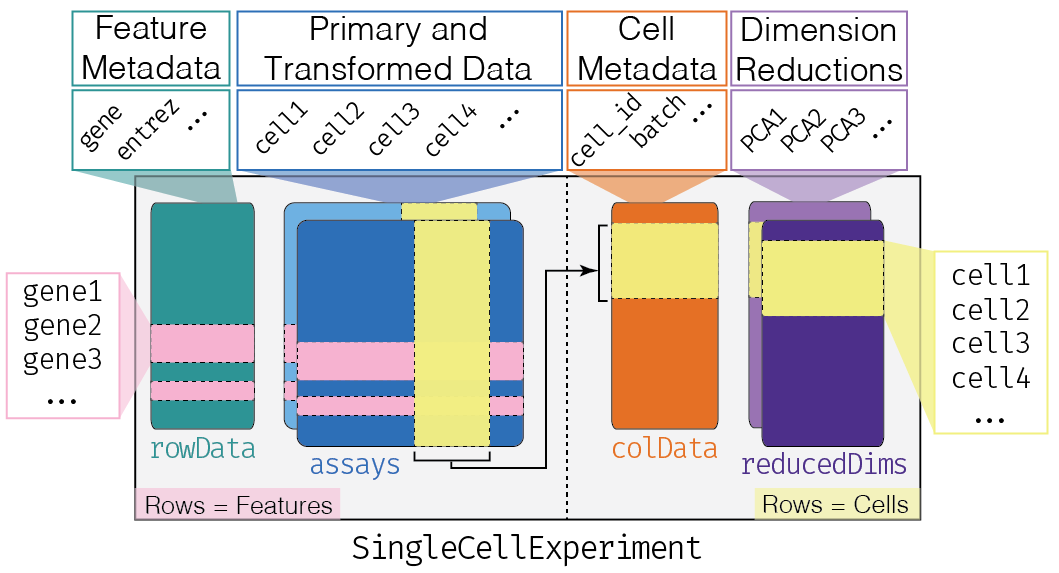
Refer to the Bioconductor OSCA book for more information on
- https://bioconductor.org/books/OSCA.intro – Loading data and working with SingleCellExperiment objects
- https://bioconductor.org/books/OSCA.basic – Analyzing data from normalization to marker gene detection
Use help() to display the built-in R Documentation for a given function (e.g. help(sort)) after loading the appropriate package
Exercises
There are three exercises in this assignment:
- Load the Fly Cell Atlas Gut data and inspect the counts and cell metadata
- Explore the data at the gene-level, cell-level, and percent of mitochondrial reads
- Identify marker genes for subsets of epithelial cells and somatic precursor cells
Before you do anything else, create an R script for this assignment. Everything you’ll need to do for this assignment will be done in this script and submitted via GitHub. Use comments to separate your code blocks into Exercise 1, Step 1.1; Exercise 1, Step 1.2; etc., and include written answers to questions as comments. You must show your work to receive full credit for each question by providing the code you used.
1. Load data
Load packages
Load the following packages using library()
- zellkonverter – loads single cell data from the popular H5ad file format and creates a SingleCellExperiment object
- scuttle, scater, and scran – provide core single cell functionality
- ggplot2 – data visualization
Load and inspect data (0.5 pt)
Load Gut data from flycellatlas.org
- Download the Gut “10x, RAW, H5AD” data using a web browser (should be 612 MB)
- Create a SingleCellExperiment object named
gutby loadingv2_fca_biohub_gut_10x_raw.h5adusingreadH5AD()from zellkonverter - Change the assay name from
XtocountsusingassayNames(gut) <- "counts" - Normalize counts using
gut <- logNormCounts(gut)
Question 1: Inspect the gut SingleCellExperiment object (0.5 pt)
- How many genes are quantitated (should be >10,000)?
- How many cells are in the dataset?
- What dimension reduction datasets are present?
Inspect cell metadata (0.5 pt)
Question 2: Inspect the available cell metadata (0.5 pt)
- How many columns are there in
colData(gut)? - Which three column names reported by
colnames()seem most interesting? Briefly explain why. - Plot cells according to
X_umapusingplotReducedDim()and colouring bybroad_annotation
2. Explore data
Explore gene-level statistics (1 pt)
Sum the expression of each gene across all cells
- Create a vector named
genecountsby usingrowSums()on the counts matrix returned byassay(gut)
Question 3: Explore the genecounts distribution (1 pt)
- What is the mean and median genecount according to
summary()? What might you conclude from these numbers? - What are the three genes with the highest expression after using
sort()? What do they share in common?
Explore cell-level statistics (1 pt)
Question 4a: Explore the total expression in each cell across all genes (0.5 pt)
- Create a vector named
cellcountsusingcolSums() - Create a histogram of
cellcountsusinghist() - What is the mean number of counts per cell?
- How would you interpret the cells with much higher total counts (>10,000)?
Question 4b: Explore the number of genes detected in each cell (0.5 pt)
- Create a vector named
celldetectedusingcolSums()but this time onassay(gut)>0 - Create a histogram of
celldetectedusinghist() - What is the mean number of genes detected per cell?
- What fraction of the total number of genes does this represent?
Explore mitochondrial reads (1 pt)
Sum the expression of all mitochondrial genes across each cell
- Create a vector named
mitoof mitochondrial gene names usinggrep()to searchrownames(gut)for the pattern^mt:and settingvalueto TRUE - Create a DataFrame named
dfusingperCellQCMetrics()specifying thatsubsets=list(Mito=mito) - Confirm that the mean sum and detected match your previous calculations by converting
dfto a data.frame usingas.data.frame()and then runningsummary() - Add metrics to cell metadata using
colData(gut) <- cbind( colData(gut), df )
Question 5: Visualize percent of reads from mitochondria (1 pt)
- Use
plotColData()to plot thesubsets_Mito_percenton the y-axis against thebroad_annotationon the x-axis rotating the x-axis labels usingtheme( axis.text.x=element_text( angle=90 ) )and submit this plot - Which cell types may have a higher percentage of mitochondrial reads? Why might this be the case?
3. Identify marker genes
Analyze epithelial cells (3 pt)
Question 6a: Subset cells annotated as “epithelial cell” (1 pt)
- Create an vector named
coithat indicates cells of interest whereTRUEandFALSEare determined bycolData(gut)$broad_annotation == "epithelial cell" - Create a new SingleCellExperiment object named
epiby subsettinggutwith[,coi] - Plot
epiaccording toX_umapand colour byannotationand submit this plot
Identify marker genes in the anterior midgut
- Create a list named
marker.infothat contains the pairwise comparisons between all annotation categories usingscoreMarkers( epi, colData(epi)$annotation ) - Identify the top marker genes in the anterior midgut according to
mean.AUCusing the following code
chosen <- marker.info[["enterocyte of anterior adult midgut epithelium"]]
ordered <- chosen[order(chosen$mean.AUC, decreasing=TRUE),]
head(ordered[,1:4])
Question 6b: Evaluate top marker genes (2 pt)
- What are the six top marker genes in the anterior midgut? Based on their functions at flybase.org, what macromolecule does this region of the gut appear to specialize in metabolizing?
- Plot the expression of the top marker gene across cell types using
plotExpression()and specifying the gene name as the feature andannotationas the x-axis and submit this plot
Analyze somatic precursor cells (3 pt)
Repeat the analysis for somatic precursor cells
- Subset cells with the broad annotation
somatic precursor cell - Identify marker genes for
intestinal stem cell
Question 7: Evaluate top marker genes (3 pt)
- Create a vector
goithat contains the names of the top six genes of interest byrownames(ordered)[1:6] - Plot the expression of the top six marker genes across cell types using
plotExpression()and specifying thegoivector as the features and submit this plot - Which two cell types have more similar expression based on these markers?
- Which marker looks most specific for intestinal stem cells?
Submit
Submit an R script with your code and answers to questions for
- Loading data (1 pt)
- Exploring data (2.5 pt)
- Identifying marker genes (3 pt)
Be sure to include the following plots
- Broad annotation vs % mitochondrial reads (0.5 pt)
- Epithelial cells UMAP colored by annotation (1 pt)
- Expression plot of top marker gene in epithelial cells according to annotation (1 pt)
- Expression plot of top six marker genes in precursor cells according to annotation (1 pt)